IRB & Cayuse FAQs
IRB stands for Institutional Review Board. It is a committee at TCU that reviews and makes determinations on research that involves human subject participants. Check out the Human Subject Research webpage to learn more.
No, only human subject research gets reviewed by the TCU IRB committee. Other types of research such as animal or biosafety, are reviewed by their own respective committees. However, if Biosafety or IACUC committee approval is required, you must obtain that committee’s approval before IRB approval. For your reference please review the Laser Safety, Biosafety, or Animal Care and Use webpages to learn more.
The Office of Research Compliance (ORC) handles and coordinates research compliance at TCU which is conducted under the purview of the federal regulations.
ORC Staff are available to answer any questions concerning TCU researchers meeting professional, regulatory, and university requirements for their IRB projects.
ORC Staff can be contacted by phone or email. Click for our contact details.
Office of Research Compliance (ORC) has introduced a new online platform that handles all IRB research submissions. This system is called Cayuse Human Ethics.
Cayuse Human Ethics handles everything from submitting documents, to reviews and approvals. All current TCU faculty and staff have accounts and can log in with their TCU credentials using single sign-on. Students who want access to Cayuse will need to request an account to be made first.
Click to log in to Cayuse.
Click to Request Access to Cayuse.
As of January 10th 2022, the Office of Research Compliance will no longer be accepting applications through email. Everything will be handled through the online system, Cayuse Human Ethics.
For Exempt studies approved before January 10th 2022, you can close out your studies via IRBSubmit@tcu.edu
Once you have logged in to the home page, in the top right corner, click on Then select Human Ethics.
Click to learn how to make your Initial Submission.
External study team personnel or Non-Affiliated individuals will not have Cayuse accounts. In addition, no accounts will be created for them.
However, if the personnel are going to be collaborating on the research with you, then follow these steps:
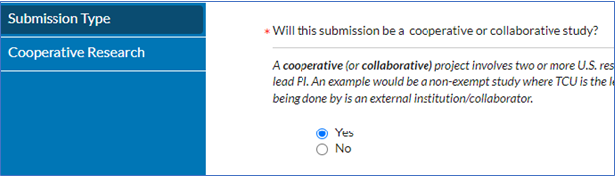
- When completing your initial submission, go to the Submission Type section> Select Yes to the below question, to which the Cooperative Research Section appears to the left> In Cooperative Research section, answer the questions
- Here you will provide information about the external personnel and their roles/ responsibility within the research.
The awaiting authorization is a safety check and prompt for the Principal Investigator (PI) to make sure everything is properly completed. Then, the PI must ‘Certify’ the submission before it moves along in the review process.
***Note: the first time submitting (regardless of the submission type i.e.: initial, modification, renewal, or incident), will need to be certified by the PI. If revisions are needed and once completed, then the co-investigators can certify the submissions themselves.
To do this, the PI needs to log into Cayuse > access their dashboard> click on the study name itself under My Tasks or the tile that says Awaiting Authorization > then click on the blue button to Certify.


This status indicates that the Office of Research Compliance (ORC) staff is conducting pre-review checks on the submission; to ensure all necessary information is provided and completed appropriately.
An IRB Analyst will be assigned, determine the review level for the protocol, and begin a preliminary review. The Analyst may return a submission if they need additional information or clarification before sending the protocol to review.
This status indicates that the submission has gone through a pre-review check and there are revisions or questions (comments) that need to be addressed. These will be indicated by speech bubbles on the menu sidebar to the left. The speech bubble will denote the number of areas that need attention within each section.



Comments will appear as little black speech bubbles in the section that needs attention. Click on it to expand the comment. Address the question or make the necessary revision. You can also reply directly to the comment.
Once completed, then click on the red toggle and select Address (which turns green to mark as complete).
This status indicates that submission has completed the pre-review check and currently under review by the TCU IRB committee.
Regardless of the level of review or number of board members reviewing, this status indicates that the IRB review process is underway. For Full Board reviews, this can be expected to go through the Board’s next meeting date, unless the submission has already been through a Full Board Review and needs only minor edits.
This letter indicates that the submission went under review by the IRB committee and further revisions or questions were recommended. These will be indicated as comments within the submission, for the study team to update/ edit and address. Once completed, the submission can be sent back for final approval, if the changes are satisfactory to the IRB committee.
Once you have logged into your Dashboard, go to the Submission Details of your study and click on the Attachments tab at the bottom. Use only the documents that have been labelled with the respective IRB number and have been stamped with the blue icon (to download, click on the three dots next to the file).

Click to learn how to make your Modification Submission.
For studies that were approved by the Expedited process, require an Annual Check-in; studies approved by the Full Board, require a Continuing Review. Both instances can be addressed by submitting a Renewal and filling out the information correctly based on the approval status. Click to learn how to make a Renewal Submission.
Any instances that occur because of or related to the research, whereby affecting the participants in any manner (injuries, data or confidentiality breaches, and/ or unexpected risks), need to be disclosed to the IRB. Click to learn how to make an Incident Report.
There are two types of statuses in Cayuse Human Ethics to help you keep track of project progress:
- Study Status: Represents the overarching place of a project in the lifecycle
- Submission Status: Shows where the current IRB request regarding your protocol is at
Submission Statuses
Unsubmitted: An in-draft submission that is open for editing and has not yet been routed to the IRB. Likely some required items still need to be completed before the submission can be declared complete and certified.
Awaiting Certification: Once a submission is declared complete, it informs the certifying researchers that they need to sign off on it. A submission cannot be edited once it reaches this point unless a certifier rejects it.
Awaiting Org Approval: Once all certifications are done, department approvers will be notified that they need to sign off on it. This is the last step before the IRB Office gets to see the submission unless an Organizational Approver reopens it for editing by rejecting the submission.
Reopened: This status is applied if a Certifier, Approver, Analyst, or Reviewer returns the submission at any point. It is functionally the same as the Unsubmitted status.
Under Pre-Review: Once all certifications and approvals are in, the submission routes to the IRB Office where an IRB Analyst will be assigned, determine the review level for the protocol, and begin a preliminary review. The Analyst may return a submission if they need additional information or clarification before sending the protocol to review.
Under Review: Regardless of the level of review or number of board members reviewing, this status indicates that the IRB review process is underway for a submission. For Full Board reviews, this can be expected to go through the Board’s next meeting date, unless the submission has already been through a Full Board Review and needs only minor edits.
Under Post Review: This status is applied once the Reviewer’s assessment of a submission is completed and they have come to a decision to either approve the protocol or ask for revisions. After a decision is made, the submission routes back to the assigned IRB Analyst for a final check before declaring the review complete, returning it, sending it to additional Reviewers (if needed).
Review Complete: Once an approval or exemption decision has been reached for a protocol and the IRB Office has signed off on it, this status becomes permanent for the submission and the Study Status may change to reflect it, as when an Initial Submission is first declared “Approved” or when an expired protocol finishes the continuing review process.

